About the programme
If you are fascinated by organic chemistry and the design and synthesis of new drug substances, then the programme might be of interest to you. It is an excellent springboard to a research-based career in academia and industry as you do an extended thesis project getting hands-on experience. You are likely to find work in the pharmaceutical, biopharmaceutical and biotech industry that conducts research to develop new drugs.
What makes the programme at UCPH unique?
The programme offers you the opportunity to tailor an academic profile during your studies. Through elective courses and an extended thesis project you can specialise in an area of interest. You will join an international study environment, and the education and projects are closely connected to established research groups both at the university and industry giving you a strong network.What specialisations does the field of study offer?
You will tailor a two-year master’s programme focused on organic chemistry and design and synthesis of druglike molecules – both small molecules and biopharmaceuticals. The programme offers great flexibility, which enables you to create a specialised academic profile. This could be in the fields of organic chemistry, drug discovery, biopharmaceuticals, computational methods, structure-based drug design or radiopharmaceutical chemistry.Considering studying at UCPH this September?
Apply by 15 January if you are an applicant from outside the EU/EEA/Switzerland. Apply by 1 March if you are from the EUAdmission and application
To apply for admission to this master's degree programme, you must have completed a qualifying bachelor’s degree or a similar Danish or international degree programme which is assessed to be relevant. Apply for admission via the application portal.
Below, you can read more about admission requirements and which documents to upload in the application portal.
Academic admission requirements
Here you'll find the different academic requirements depending on which qualifying degree you hold.
You have legal right of admission if you have a
- Bachelor’s degree in Medicinal Chemistry from the University of Copenhagen
This means that you are guaranteed a place on the master’s programme in Medicinal Chemistry if you apply in time to begin within 3 years after the completion of your bachelor’s degree.
You meet all academic requirements if you hold one of the degrees listed below:
- Bachelor's degree in Physics
- Bachelor of Science in Pharmacology
- Bachelor’s degree in Chemistry with a specialisation in Medicinal Chemistry from the University of Copenhagen
- Bachelor’s degree in Pharmacy from the University of Copenhagen, including:
- the course NKEA05040U Advanced Organic Chemistry [Videregående organisk kemi (VO)]
- a bachelor’s project in Pharmaceutical Sciences [course code SFABIL110U] within the scientific disciplines of medicinal chemistry and/or experimental organic chemistry
Note, that having a bachelor’s degree that automatically fulfil the admission requirements does not guarantee you admission to the programme.
You must hold a bachelor’s degree in chemistry, medicinal chemistry or pharmacy and have accumulated credits in chemical and biological disciplines.
In your bachelor’s degree you must have accumulated:
- At least 80 ECTS credits on chemistry courses in the fields of organic and physical chemistry with the emphasis on theoretical and experimental chemistry courses
- At least 30 ECTS credits from biology courses in the fields of biochemistry, molecular biology, physiology and pharmacology, of which at least 5 ECTS credits must come from within general pharmacology
Note, that having a bachelor’s degree that automatically fulfil the admission requirements does not guarantee you admission to the programme.
You must have earned your bachelor’s degree within a maximum of 5 years prior to the start of the first semester of the master’s programme, e.g. for the intake in the autumn of 2026, you must have graduated by September 2021 or thereafter. In exceptional circumstances the Admissions Committee may waive the graduation year requirement.
If your bachelor’s degree is too old, you can apply for an exemption from the graduation year requirement. If you choose to apply for an exemption, you must submit the following documents along with your application for admission:
- A letter explaining how you have maintained your academic qualifications since graduation (e.g. relevant work, internships, further studies)
- Relevant documentation (e.g work contracts, diplomas etc).
If you already have a master's degree from Denmark or another country, you can, in principle, only be admitted to a new degree programme if there are places available on the programme for which you are applying for admission.
When we assess whether you meet the admission requirements for the master's degree program, Danish legislation only allows us to assess your bachelor's degree. Consequently, you cannot study supplementary courses between bachelor's and master's degree programs in order to meet the admission requirements.
If you have passed courses/projects before you complete the qualifying bachelor's degree, these can be included in the assessment, even though they are not part of the bachelor's degree program.
- It applies to courses/projects you have taken as single subjects and courses/projects you have taken as part of another study program.
- A maximum of 30 ECTS credits of these courses/projects may be included.
Language requirements
You are required to document that you fulfil the language requirement English B, unless you have a legal right of admission to the programme you are applying for.
Please note that you must have the documentation ready by the application deadline.

Application deadlines
Study start in September
1 March at 23:59
Application deadline for Danish applicants and applicants from within the EU, EEA and Switzerland.
Open for applications from 16 January. You will receive a reply by 10 June.
15 January at 23:59
Application deadline for applicants from outside the EU, EEA and Switzerland.
Open for applications from 15 November. You will receive a reply by 13 March.
How to apply
Choose your category and read how you apply for admission. You can also find information about deadlines and documentation requirements.
Please note that you must also select according to your citizenship:
- Citizen from Denmark, EU, EEA or Switzerland (EU)
- Citizen from countries outside EU, EEA or Switzerland (NON-EU)
How we assess your application
The programme accepts a maximum of 50 students.
If the number of qualified applicants to the programme exceeds the number of places available qualified applicants will be prioritised according to the following criteria:
- Grade point average from the bachelor’s degree
- Grade point average from courses in organic chemistry (including physical chemistry) and biological courses (including fields of biochemistry, molecular biology, physiology, and pharmacology)
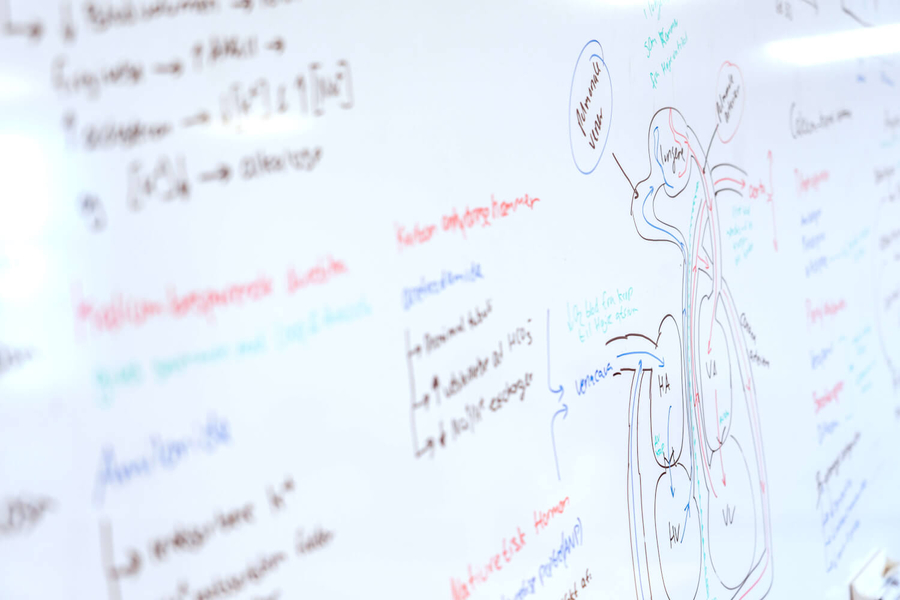
| Admission statistics Medicinal Chemistry 2025 | |
|---|---|
| Admitted (of which have start in February) | 46 (0) |
| Admission distribution (legal right/other) | 59% / 41% |
| Applicants | 94 |
| Age average | 25 |
| Nationality (dk/international) | 59% / 41% |
Programme structure
The MSc in Medicinal Chemistry is a 2-year programme taught in English and there are approximately 40 students in a year.
The programme is known for its cooperation with industry, since there is a close concentration of biotech and pharmaceutical companies in the region. Medicon Valley, as the hub is called, is home to almost 300 ‘life science’ companies.
Teaching forms are lectures, classroom teaching, computer exercises, laboratory work and research projects.
Depending on your choice of study plan, these are the approximate numbers.
- 40 % mandatory courses
- 10-35 % elective courses
- 25-50 % thesis
The first year consists of five compulsory courses and electives. In the second year, you can tailor a specialised academic profile, as you take elective courses and write your thesis.

1st year
| Blok 1 | Blok 2 |
|---|---|
| Reactions and Synthesis in Medicinal Chemistry (15 ECTS) | Reactions and Synthesis in Medicinal Chemistry (15 ECTS) - continued |
| Biopharmaceuticals: Design and Modification of Biomacromolecules (7.5 ECTS) | Medicinal and Biostructural Chemistry (7.5 ECTS) |
| Blok 3 | Blok 4 |
|---|---|
| Introduction to Physical Organic Chemistry (7.5 ECTS) | Structure-based Drug Research (7.5 ECTS) |
| Elective course (7.5 ECTS) | Elective course (7.5 ECTS) |
2nd year
| 1st semester | 2nd semester |
|---|---|
| Elective course (up to 15 ECTS) or Master's thesis (45, 52.5 or 60 ECTS) | Master's thesis (45, 52.5 or 60 ECTS) - possibly continued |
Programme Curriculum
If you are more interested in the academic content, regulations, and examination requirements, you should consult the curriculum, which serves as the legal foundation for the programme.
There is both a curriculum specific to each degree programme and a general curriculum that applies across the faculty.
Please note that curricula are often revised annually. Any new versions will be published no later than during the spring semester.
The first year consists of five compulsory courses within the following subject areas: medicinal chemistry, structure-based drug discovery, advanced organic chemistry, and biopharmaceuticals - core subjects in modern drug discovery research.
If you wish to specialise within a certain area of medicinal chemistry, you can choose to focus your study programme, giving you an academic profile.
Your profile could be within one of the areas listed below or another tailor-made combination of elective courses and your thesis project, which align with your competences and interests.
Your elective courses are not limited to these profiles and could also include data science or other areas of your interest.
Profile in Synthetic Organic Chemistry
With this profile you will be a specialist in synthesizing druglike molecules – that is, the actual active pharmaceutical ingredients (APIs). The majority of new APIs are still small molecules with a molecular weight of less than 500 Da. These small molecules are most often made by advanced chemical synthesis methods; hence a thorough knowledge of organic chemistry is needed to become a skilled synthetic medicinal chemist.
You can take advanced chemistry elective courses to develop your competences within theoretical and experimental organic chemistry. These competences might be very useful for you as a future medicinal chemist working with drug discovery or scale-up in drug development departments.
Profile in Biopharmaceuticals
If you would like a specialised focus on peptide- and protein-based drugs and other biopharmaceuticals, you can specialise in biopharmaceuticals.
The specialisation consists of a number of elective courses with relation to biopharmaceuticals. You will get insights into discovery and early development of biopharmaceuticals. Additionally, you will learn how these groups of drug molecules can be used to solve questions on the borderline between chemistry and biology or be used as potential drug compounds.
Profile in Structure-Based Drug Design
Almost all drugs interact with proteins, which are structurally very diverse and have many different functions. Since proteins are involved in almost all biological processes that sustain life and diseases, they are natural drug targets. Hence, it is possible to treat the symptoms of disease by using drugs that change protein activity. That may sound simple, but in practice it is a major challenge.
A good starting point in the production of new drugs is to elucidate the three-dimensional structure of the target molecule. The drug’s role is to open or close the functionality of the protein. Medicinal chemists can use this knowledge to design drugs.
Profile in Radiopharmaceutical Chemistry
The profile consists of elective courses that will develop your competences within radiopharmaceutical/nuclear chemistry research, development, and production processes.
The profile in radiopharmaceutical chemistry is established in a close collaboration between Rigshospitalet (the university hospital in Copenhagen) and the Department of Drug Design and Pharmacology at the University of Copenhagen.
As part of your electives, you can choose to do a short internship or research project (called an individualised study unit) either at research groups at the University of Copenhagen or at, for instance, a medical company. Your lecturers may have academic input and contacts, but please note that finding the place and supervisor for this is on your own initiative.
It is possible to study abroad during your degree. Your third semester is best suited for studying abroad but it will require a modified study plan. Alternatively, you can choose to write part of your thesis abroad. It is also possible to take a summer course in place of an elective.
Why Study Abroad?
A main objective of studying abroad is to further widen your academic knowledge and network. You are also likely to benefit socially and culturally.
It is a good idea to seek advice from lecturers and the student guidance when planning your studies abroad to find out where to go and how to structure your academic programme. Your lecturers may have academic input, international contacts, and may also be able to provide you with references which can prove useful.
Exchange Agreements
The University of Copenhagen has an extensive number of exchange agreements with universities worldwide.
Information about partner universities specific to Medicinal Chemistry is available through the International Relations Office at the Faculty of Health and Medical Sciences.
Student Mobility at the Faculty of Health and Medical Sciences
Researchers at the Faculty of Health and Medical Sciences and the Faculty of Science offer a wide range of thesis subjects.
You can carry out your thesis project in a research group at the Department of Drug Design and Pharmacology, the Department of Chemistry or any other relevant department at the university. Also, there are opportunities to write an industry-based thesis in Denmark or abroad.
All research groups involved in the programme enjoy close cooperation with relevant medicinal chemistry departments in pharmaceutical companies of Medicon Valley. This cooperation is a key element of several programme courses as well as thesis-related work. The close contact established during the programme is also expected to play an important role in the transition from study to professional career.

Career opportunities
Upon completion of the master’s programme, you will obtain the title Master of Science in Medicinal Chemistry.
The educational profile is in high demand, because the programme is pharmaceuticals-oriented and focuses on medicines and the development of drugs and related products.
You can expect to find work in the pharmaceutical, biopharmaceutical and biotech industry that conducts research into new drugs. In the industry, you will work with other research scientists in the drug field to design and synthesise new compounds with the potential to become future drugs.
Another important job area for medicinal chemists is working with patent experts and others to ensure that new drugs are patented.
Knowledge
When graduating, you will have acquired knowledge about:
- The rational basis for design and development of drugs
- New and effective methods of synthesis for incorporation of the most important functional groups
- The relationship between molecular structure and biological activity at the molecular level, including the importance of steric, stereochemical, conformational and electrostatic factors
- Structural chemical methods that can be used in the rational design of drugs
- Solid-phase methods of synthesis used to make peptides and peptide derivatives, including peptidomimetics
- The significance of conformational, steric and electronic factors with regard to regio- and stereoselective syntheses of drug candidates
- Physical-chemical parameters important for the development of potential drug substances.
Skills
You are able to:
- Analyse and evaluate methods of synthesis to choose an optimal strategy for the synthesis of a target molecule
- Design, plan, and conduct advanced syntheses on the basis of a critical review of articles in international journals and patent literature
- Use and critically evaluate results achieved by modern computer-based methods for structural-activity analyses of biologically active compounds (potential drugs)
- Explain the key principles used for the rational basis of design and development of new drugs
- Explain the most important chemical, physical-chemical and pharmacokinetic properties of important groups of drugs
- Explain the properties and reactivity of heteroaromatic compounds
- Plan chemical modifications of proteins and estimate the effects.
Competences
You are able to:
- Plan, carry out and report on research and development projects. For example, related to the design and production of new small molecule and macromolecular drugs in cooperation with scientists from other disciplines
- Plan and conduct advanced organic chemical syntheses as well as syntheses and modifications of peptides, proteins etc. relevant to the pharmaceutical and biotechnological industries.
Your qualifications as a Medicinal Chemistry graduate are applicable in other contexts in the pharmaceutical, biopharmaceutical, and biotechnical industries, for example, in connection with the design, production, and development of potential new drugs.
Examples of employment opportunities
- PhD programmes and industry science positions within organic chemistry, medicinal chemistry, and drug discovery.
- Development of new radioactive entities including routine production of radiopharmaceuticals for diagnosis and therapy at hospitals and pharmaceutical companies.
- Patenting such as positions in pharmaceutical companies and private- or public-sector patent agencies.
- Developing effective, safe, robust synthesis procedures for use in high-volume production of the active drug substance.
- Chemistry and manufacturing control (CMC) as an employee of a parent company or a Contract Research Organisation (CRO).
- Regulatory affairs and Quality Assurance/Quality Control (QA/QC).
The University of Copenhagen is located in the midst of one of the world’s leading biotech areas – Medicon Valley – home to almost 300 ‘life science’ companies. The School of Pharmaceutical Sciences is known for its collaboration with industry.
One of these collaborative endeavours is the school’s own Drug Research Academy (DRA). DRA covers all key research areas in drug development from research, development and production to clinical testing.
This close concentration of biotech and pharmaceutical companies in the region coupled with the school’s collaboration with industry provides good opportunities for students to build contacts to future employers during their studies.
There are excellent opportunities to find student jobs related to the field and to initiate thesis projects at one of the many companies in the area.
Student life
When you study medicinal chemistry, you will be part of an international and vibrant study environment. You can join a lot of clubs, extracurricular activities and social events. You also follow courses with students from other programmes and meet up with your fellow students in the student cafe.
PharmaSchool has won awards for best study environment and older students make an effort to welcome new students to PharmaSchool.
You will primarily be studying at the University of Copenhagen’s North Campus.

Contact student guidance
Questions about study choice and admission
Our student guidance are ready to assist you with answers to your questions about:
- application procedure and the digital application portal
- admission- and language requirements
- documentation
- study life
- career opportunities
- study choice or doubts
Did you not find what you were looking for?
You can find answers to questions most often asked by potential students in the FAQ. Read the FAQ
Questions about the digital application-portal?
Do you have questions about digital application? Check our user guide to the application portal.
In case of technical problems, please contact the IT-support by
- Mail: it-service@adm.ku.dk // Tel: +45 35 32 32 32
Location
- North Campus, Universitetsparken 5, DK-2100 København Ø.